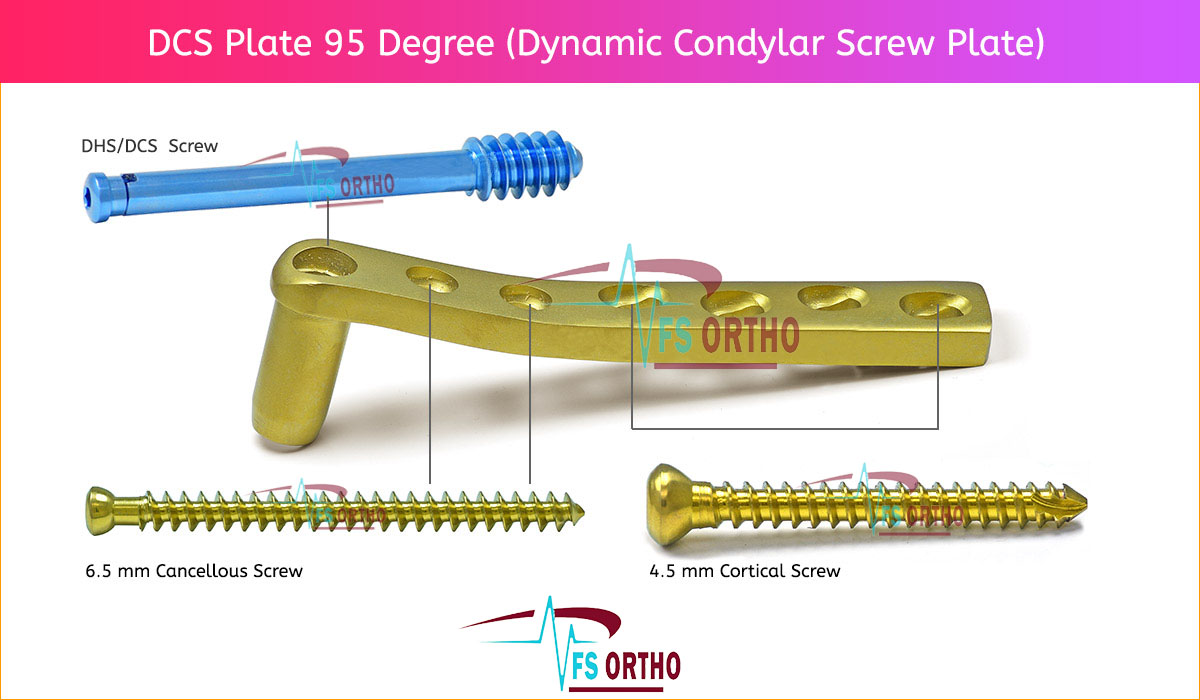
DCS Plate (Dynamic Condylar Screw Plate) is designed to provide strong and stable internal fixation of certain distal femoral and subtrochanteric fractures, with minimal soft tissue irritation.
DCS Plate (Dynamic Condylar Screw Plate) Specification
⦁ Plates are made of 316L stainless steel and Titanium.
⦁ The ⦁ DCS are available with 6, 7, 8, 10, 12, 14, 16, 18, and 20 holes, for varied clinical situations.
⦁ The number of screw holes per plate length is maximized, without compromising plate strength. This allows an increased number of fixation points with a smaller incision.
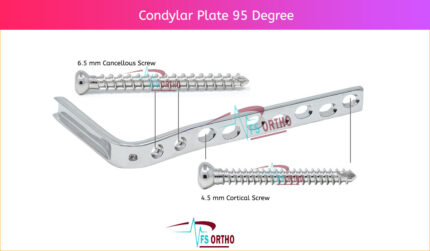
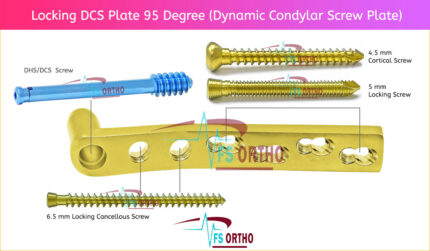
⦁ The two holes closest to the barrel accept 6.5 mm cancellous bone screws. This enhances stability by allowing additional fixation of the most distal condylar fracture fragments or fixation of the most proximal subtrochanteric fracture fragments.
⦁ DCP holes in the DCS side plate allow angulation of 4.5 mm cortex screws and axial compression across a shaft fracture.
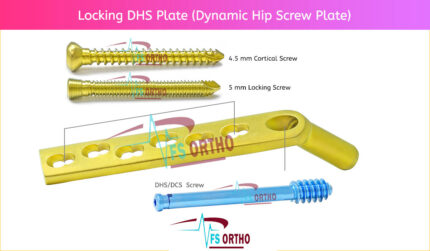
⦁ The DHS/DCS lag screw is available in 50 mm to 145 mm lengths. The DHS/DCS compression screw can be used for additional compression; only one
size compression screw is needed.
⦁ Two flats within the DCS plate barrel correspond to the two-flat design of the lag screw, preventing rotation of the DHS/ DCS lag screw within the barrel. The two-flat design also eases insertion of the plate over the DHS/DCS lag screw.
⦁ The DHS plates have a low-profile design, reducing the risk of iliotibial band irritation (distal femoral fractures) and trochanteric bursitis (subtrochanteric fractures).
⦁ The DCS instruments also provide direct measurements throughout the DCS procedure, allowing proper reaming, tapping, and lag screw insertion depth. The built-in stop and locking nut on the DCS triple reamer prevent over-reaming.
⦁ General Instruments are available for DCS Plate such as Plate Bending Press, Plate Holding Forceps, Plate Bending Pliers, Bone Holding Forceps, Bone Elevators, Bone Cutter, Bone Nibbler, Depth Gauge, Sleeve, Screw Driver, Trocar Sleeve etc.
DCS (Dynamic Condylar Screw Plate) Uses
The DCS Plate is indicated for the following fractures of the proximal distal:
⦁ Intercondylar fractures
⦁ Supracondylar fractures
⦁ Unicondylar fractures
The DCS Plate is indicated for the following fractures of the proximal femur:
⦁ Transverse subtrochanteric fractures
⦁ Short oblique subtrochanteric fractures
⦁ Long oblique subtrochanteric fractures
DCS Plate Contraindications
Plate is contraindicated for use in areas with active or latent infection or insufficient quantity or quality of bone.
DCS Plate Precautions
⦁ Confirm functionality of ⦁ instruments and check for wear during reprocessing. Replace worn or damaged instruments prior to use.
⦁ It is recommended to use the instruments identified for this plate.
⦁ Handle devices with care and dispose worn bone cutting instruments in a sharps container.
⦁ Always irrigate and apply suction for removal of debris potentially generated during implantation or removal.
Dynamic Condylar Screw Plate Warnings
⦁ Plate can break during use (when subjected to excessive forces). While the surgeon must make the final decision on removal of the broken part based on associated risk in doing so, we recommend that whenever possible and practical for the individual patient, the broken part should be removed. Be aware that implants are not as strong as native bone. Implants subjected to substantial loads may fail.
⦁ Instruments, screws and cut plates may have sharp edges or moving joints that may pinch or tear user’s glove or skin.
⦁ Take care to remove all fragments that are not fixated during the surgery.
⦁ While the surgeon must make the final decision on implant removal, we recommend that whenever possible and practical for the individual patient, fixation devices should be removed once their service as an aid to healing is accomplished. Implant removal should be followed by adequate post-operative management to avoid refracture.
General Adverse Events
As with all major surgical procedures, risks, side effects and adverse events can occur. While many possible reactions may occur, some of the most common include: Problems resulting from anesthesia and patient positioning (e.g. nausea, vomiting, dental injuries, neurological impairments, etc.), thrombosis, embolism, infection, nerve and/or tooth root damage or injury of other critical structures including blood vessels, excessive bleeding, damage to soft tissues incl. swelling, abnormal scar formation, functional impairment of the musculoskeletal system, pain, discomfort or abnormal sensation due to the presence of the device, allergy or hypersensitivity reactions, side effects associated with hardware prominence, loosening, bending, or breakage of the device, mal-union, non-union or delayed union which may lead to breakage of
the implant, reoperation.

Reviews
There are no reviews yet.