The aim of surgical fracture treatment with T Plate Craniomaxillofacial is to reconstruct the bony anatomy and restore its function. According to the AO, internal fixation is distinguished by anatomical reduction, stable fixation, preservation of blood supply, and early, active mobilization.
NAME Features and Benefits
Wide offering of T Plate geometries, sizes, and strengths
Seven screw diameters to choose from: 1.5 mm, 2 mm, 2.5 mm, 2.8 mm, 2 mm Locking, 2.5 mm Locking and 2.8 mm Locking
Rounded edges on plates for less irritation to soft tissue, where applicable
Reduced plate/screw profile, where applicable
HOLES
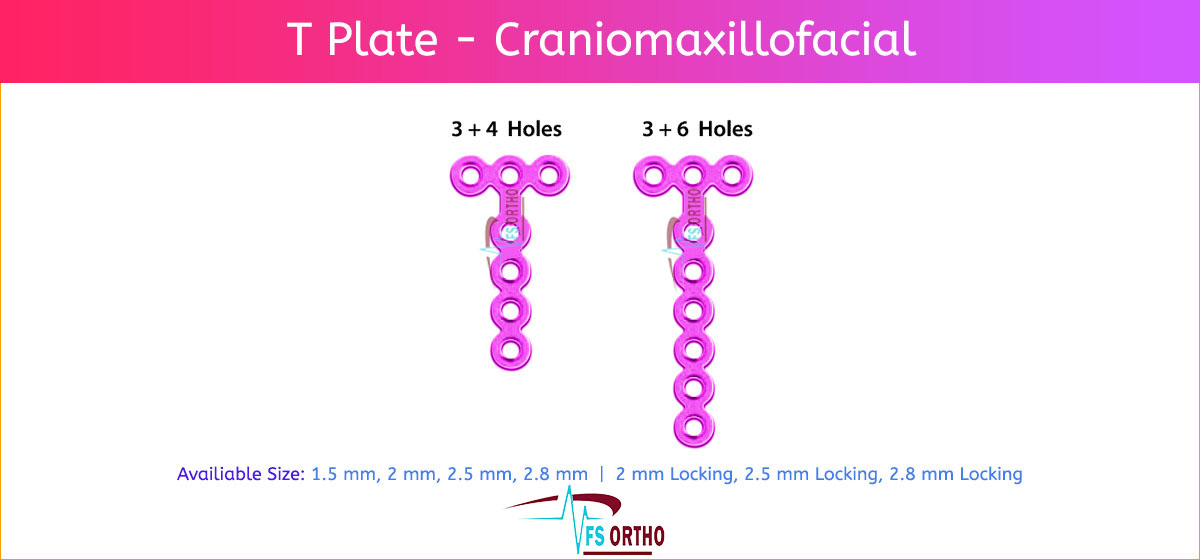
1.5 mm 3 + 4
2 mm 3 + 4
2.5 mm 3 + 4
2.8 mm 3 + 4
2 mm Locking 3 + 4
2.5 mm Locking 3 + 4
2.8 mm Locking 3 + 4
1.5 mm 3 + 6
2 mm 3 + 6
2.5 mm 3 + 6
2.8 mm 3 + 6
2 mm Locking 3 + 6
2.5 mm Locking 3 + 6
2.8 mm Locking 3 + 6
Emergency screws available for each screw diameter
Plates and Screws are made from pure Titanium
Standardized instrumentation
locking plate increases construct stability, decreases risk of screw back-out and subsequent loss of reduction. It also reduces the need for precise anatomic plate contouring and minimizes the risk of stripped screw holes.
T Plate Craniomaxillofacial Sizes
T Plate Craniomaxillofacial Indications
Plate is intended for use in selective trauma of the midface and craniofacial skeleton, craniofacial surgery, reconstructive procedures, and selective orthognathic surgery of the maxilla and chin.
T Plate Craniomaxillofacial Contraindications
Plate is contraindicated for use in areas with active or latent infection or insufficient quantity or quality of bone.
T Plate Craniomaxillofacial Precautions
Confirm functionality of instruments and check for wear during reprocessing. Replace worn or damaged instruments prior to use.
It is recommended to use the instruments identified for this plate.
Handle devices with care and dispose worn bone cutting instruments in a sharps container.
Always irrigate and apply suction for removal of debris potentially generated during implantation or removal.
T Plate Craniomaxillofacial Warnings
Plate can break during use (when subjected to excessive forces). While the surgeon must make the final decision on removal of the broken part based on associated risk in doing so, we recommend that whenever possible and practical for the individual patient, the broken part should be removed. Be aware that implants are not as strong as native bone. Implants subjected to substantial loads may fail.
Instruments, screws and cut plates may have sharp edges or moving joints that may pinch or tear user’s glove or skin.
Take care to remove all fragments that are not fixated during the surgery.
While the surgeon must make the final decision on implant removal, we recommend that whenever possible and practical for the individual patient, fixation devices should be removed once their service as an aid to healing is accomplished. Implant removal should be followed by adequate post-operative management to avoid refracture.
T Plate Craniomaxillofacial General Adverse Events
As with all major surgical procedures, risks, side effects and adverse events can occur. While many possible reactions may occur, some of the most common include: Problems resulting from anesthesia and patient positioning (e.g. nausea, vomiting, dental injuries, neurological impairments, etc.), thrombosis, embolism, infection, nerve and/or tooth root damage or injury of other critical structures including blood vessels, excessive bleeding, damage to soft tissues incl. swelling, abnormal scar formation, functional impairment of the musculoskeletal system, pain, discomfort or abnormal sensation due to the presence of the device, allergy or hypersensitivity reactions, side effects associated with hardware prominence, loosening, bending, or breakage of the device, mal-union, non-union or delayed union which may lead to breakage of
the implant, reoperation.







Reviews
There are no reviews yet.